
Write a brief passage describing a neutral atom of nitrogen-14 (N-14). The symbol for the proton is either p or p+. Neutrons are neutrally charged particles, weighing approximately 1 atomic mass unit and located in the nucleus. Sodium (Na), the 11th element in the periodic table, is commonly found as a soft metal that reacts violently with water. But when one atom of sodium combines with one atom of chlorine, you get sodium chloride (NaCl), commonly known as. Atomic Radius The easiest way to find the number of protons, neutrons, and electrons for an element is to look at the element's atomic number on the periodic table. The first shell can only hold two electrons, while the second shell can. Block Elements are organised into blocks by the orbital type in which the outer electrons are found. Has a positive charge., I tell the number of protons contained inside an atom of an element., It takes 2000 of me to equal the mass of either of my subatomic partners., I can be found by adding up the protons and neutrons together. That is, the potassium atom has a total of nineteen electrons. Because both protons and neutrons occur in the atomic nucleus, they are collectively known. In studying the periodic table, you might have noticed something about the atomic masses of some of the elements. This number is based on Carbon-12, and as a result, Carbon-12 has an. The charge on the proton and electron are exactly the same size but opposite. Complete the table with the number of protons, electrons, neutrons, and complete chemical symbol (showing the mass number and atomic number) for each neutral atom. Find out about this classification system and its history on this expedition. Atomic number is defined as the number of protons present in the nucleus of an atom. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Therefore, there is an attraction between the nucleus and the electrons. A higher atomic weight than the one on its left. The elements in group 1 are known as the alkali metals those in group 2. where are the metals and non-metals found on the periodic table?-metals are found on the left hand side of the staircase, and non-metals on the right of it. Each atom of the element contains 19 protons. where are cations on the periodic tablepennsylvania horse racing commission be located on the Periodic Table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass. Starting in the 1940s, scientists found many new elements by colliding atoms or pieces of atoms. Chlorine (Cl), the 17th element in the periodic table, most commonly exists as a yellowish-green toxic gas. First, a quick review of what the periodic table is. Transition metals are found in the centre of the periodic table between group 2 and 3. For example : atomic number of fluorine is 9 and hence the number of protons in fluorine is 9. the number of the energy level containing the valence electrons decreases. Order of elements on historic periodic table.
WHERE ARE THE METALS LOCATED ON THE PERIODIC TABLE HOW TO
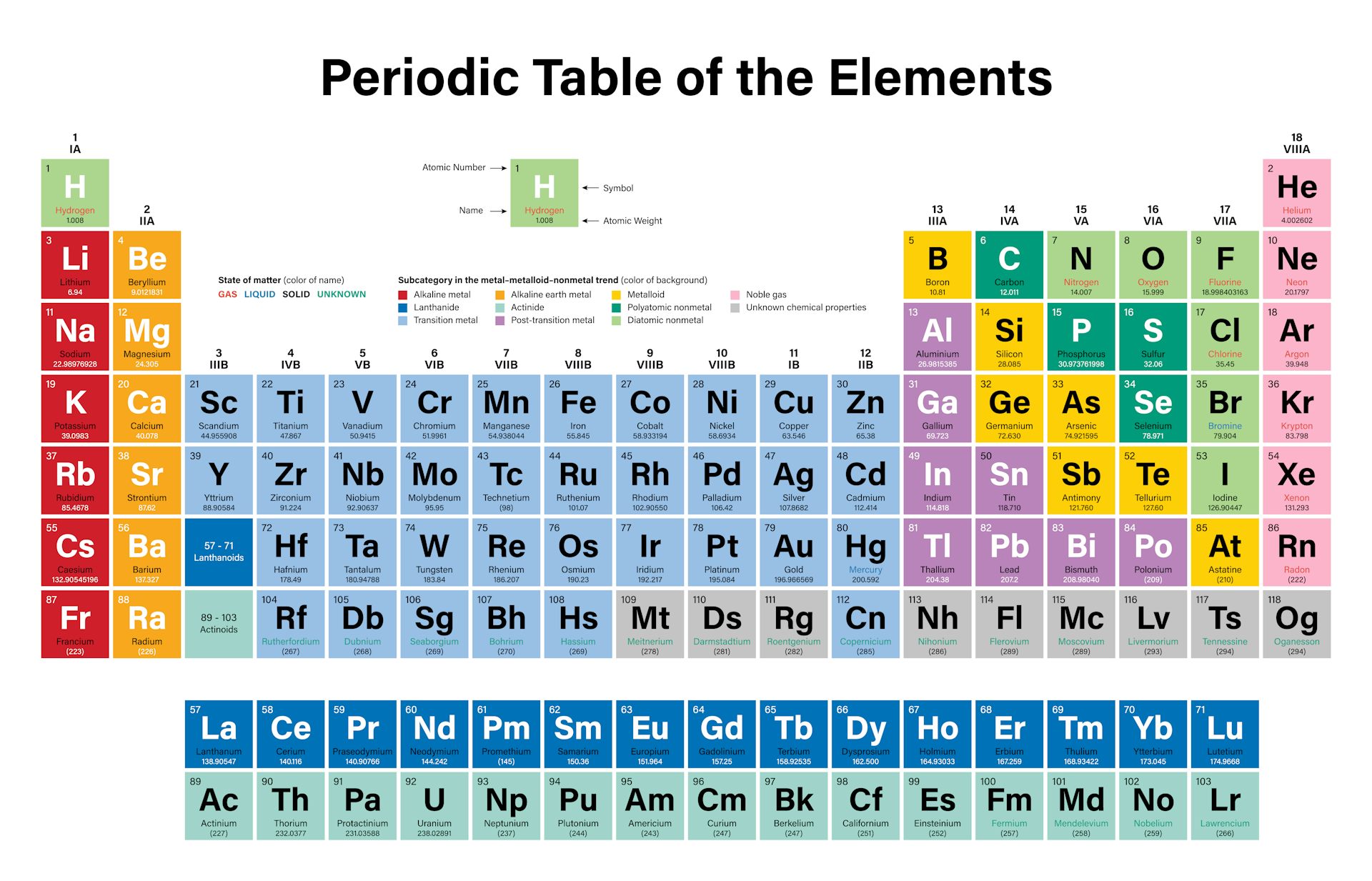
To get the most out of the table, it helps to know the parts of the periodic table and how to use the chart to predict element properties. Answer: (a) noble gas (b) chalcogen (c) alkaline earth metal (d) alkali metal. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. The atomic number of each element increases by one, reading from left to right. Study Periodic Table (The Periodic Table) flashcards.

If you look at the periodic table, you will find the metals in groups (from one to 16). subatomic particles that are located the farthest away from the nucleus- determine bonding. Group one is composed of metals that have a +1 charge, while all the metals in groups 2,3,4,5,6,7,8,9,10,11,12, and 16 have a charge +2.

Also to know is, how do you find the atomic mass on the periodic table? The mass number is found by adding the number of protons and neutrons in the atom. This version of the table, which draws on data compiled by astronomer Jennifer Johnson from Ohio State University, shows our current understanding of how each element found on Earth was originally produced. Shows all known elements in the universe.


 0 kommentar(er)
0 kommentar(er)
